Cell Assays in Alginate Hydrogel Microparticles
I develop the high throughput method to encapsulate different cellular systems into alginate hydrogel microparticles. The alginate hydrogel platform has high biocompatibility allowing continuous cell culturing and high adaptability with high speed sorting using FACS. I further apply this technology platform to address 3 biomedical challenges: engineering a model of transmitted M. tuberculosis for vaccine testing, T cell receptor identification for cancer immunotherapy and cortical neuron differentiation for drug screening.
Engineering a model of transmitted M. tuberculosis for vaccine testing
This work is in collaboration with Prof. Sarah Fortune in Harvard School of Public Health.
Mycobacterium tuberculosis (Mtb) is thought to establish infection when a few bacteria, maybe only one organism, infect and survive in an alveolar macrophage. During the earliest stages of infection, the bacterial population is at its most vulnerable at least numerically. There are a number of vaccine approaches that might allow the host to clear the organism at these early stages, by providing specificity and memory to innate immune responses. To evaluate these vaccine candidates, it is important to have an animal model in which a known, small number of organisms can be delivered reproducibly. Moreover, the physiologic characteristics of the infecting organisms should accurately reflect those of the organism transmitted in human infection.
It has been surprisingly challenging to achieve this goal, largely because of the highly hydrophobic nature of the mycobacterial cell wall, which causes the organism to form clumps that cannot be disaggregated when it is grown in broth culture. To achieve the single cell suspension of bacteria required to perform a low dose infection, the organism is typically sonicated and grown in detergent, which strips the outer leaflets of the cell wall. As these outer leaflets contain potentially important immunologic determinants, it is possible that the bacterial inoculum under standard infection protocols looks fundamentally different than the naturally transmitted organism. Even with these treatments, standardized dosing at very low inocula (1-10 bacteria) has been difficult to achieve.
Here we develop a droplet based system to trap and culture Mtb single cells in alginate droplets such that they can regenerate a complete cell wall and then be delivered in a defined, low dose inoculum in animal studies. These particles will trap and allow protected culture of individual bacteria. They can then be counted out into defined inocula, which will be stable and can be transported for animal infections. Immediately prior to infection, bacteria are released from the alginate matrix for inoculation.
Identify potent T cell receptor for cancer immunotherapy
T cell therapy brings big potential in future cancer immunotherapy. However, within each individual patient there exist a large variety of T cells each bearing a distinct T cell receptor and there is only a small subset of these T cells which can effectively kill cancer cells. We encapsulate single T cell and target cell in a 50-micron alginate hydrogel particles and isolate single potent T cells based on the functional readout from their killing and activation activities. Our technology platform will make the potent T cell receptor discovery a much faster and target specific process in the future.
Cortical neuron differentiation for drug screening
This work is in collaboration with Prof. Rubin Lee in Harvard Stem Cell Institute.
Several studies have shown that embedding stem cell spheroids in hydrogels at a certain developmental stage can make the differentiation process more controllable and efficient. For drug screening purposes, uniform differentiated tissues are desired for consistency. We scale up our microfluidics device to submillimeter dimension for this embedding procedure.
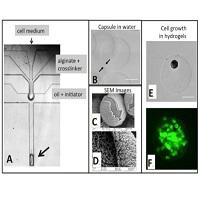
Figure 1. A: Cells in culture medium are injected into the top channel (small arrow) of a PDMS microfluidic device. This stream is surrounded by the alginate and inactive crosslinker injected in the middle channel. The oil+initiator cuts the combined aqueous stream into drops and the initiator causes alginate gelation, resulting in hydrogel particles with a cell-containing core surrounded by gelled alginate. B: Light microscopy image of a core-shell particle. The arrows indicate the alginate shell. C and D: Scanning Electron Microscopy images of coreshell particles. E: Bright-field microscopy of a single Mesenchymal Stem cell in a alginate hydrogel. F: Alginate core-shell hydrogels were created with cell occupancy of less than one Mesenchymal Stem cell per hydrogel after one week of culture.
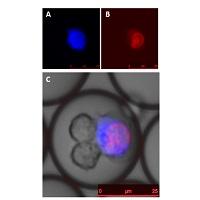
Figure 2. Natural Killer (NK) cells killing K532 cancer cell (blue) with dead dye staining nuclei (red).

Figure 3. A cortical neural spheroid encapsulated in an alginate hydrogel.
