Encapsulation and enhanced biocidal effect in biodiesel
Biodiesel is becoming more attractive due to its environmental benefits and economic feasibility. A major problem that delays wide application of biodiesel is microbial contamination. Microbes start in the interface of oil and water and then expand in the water phase. The most effective way to prevent microbial growth is to dose anti-microbial active in the water phase. However, practically people put the biocide to the storage tank, the biocide goes to the oil phase and then diffuse to the water phase. It’s hard to dose the biocide to the water directly. Due to the large oil-to-water volume ratio in the storage tank, biocide is present in less amount in water than in the oil. The extra biocide in oil will eventually be combusted and emitted into air causing air pollution.
My research is to target the biocide to the water. We use microfluidic approach to encapsulate biocide in hydrogel, which acts as a vehicle that delivers biocide to the interface of oil and water then burst release the biocide into water, leaving as little as possible biocide in the oil phase. We demonstrated the burst release of biocide upon contact with water by using fluorescein salt as a model dye molecule. Furthermore, we did biocidal experiment and compare the biocidal effect of the free biocide and encapsulated biocide.
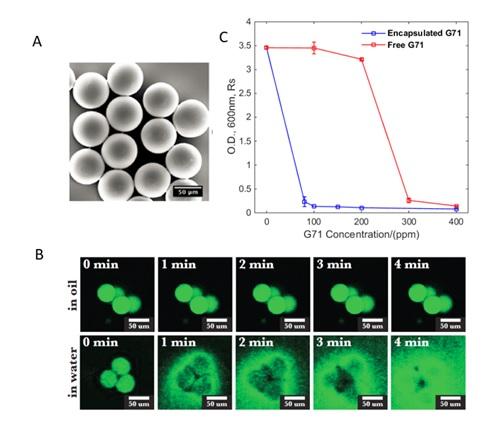
Figure1. A) Microscopic image of biocide-encapsulated hydrogel microparticles. B) Fluorescence microscopic images of fluorescein-encapsulated hydrogel microparticles in oil and in water after exposed to water for different time lengths. The green color is from the fluorescein dye. C) Remaining bacteria amount after antimicrobial treatment for 24 hours and different concentration of encapsulated Grotormar71(the biocide we used in the experiment) and free Grotomar71. The figure shows the minimum inhibitory concentration for encapsulated Grotomar71 is 80 ppm and 300 ppm for free Grotomar71.
This project is in collaboration with Feng and Ali.
Fatty amine encapsulation
Ethomeen o/12, a type of fatty amine, is a nonionic surfactant that has been widely used in the industry as emulsifier for water in oil emulsion. Meanwhile, this kind of fatty amine has another use in the industry: it’s also a very effective acid remover. It is mixed with lubricant and injected into the marine 2 stroke engine as neutralizing additive.
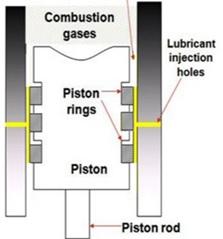
Figure 2. Schematic illustration of engine. The fuel combusts in the cylinder, pushing the piston to travel up and down. The combustion gases contain high content of acid which is highly corrosive to the engine. The lubricant mixed with fatty amine is injected through the injection holes on the cylinder wall every stroke. The piston rings spread the lubricant out to form a thin layer of lubricant oil between cylinder wall and piston. This thin layer not only helps the movement of the moving parts but also prevents the sulfuric acid generated in the cylinder corroding the engine.
However, the fatty amine is instable in high temperature. The overall goal of this project to protect fatty amines from early oxidative and thermal degradation, or at least sufficiently delay their degradation, and in the meanwhile to realize pH‐triggered release of fatty amine when it is in contact with acid. Since fatty amine itself is a surfactant, it’s hard to be encapsulated. Moreover, it’s soluble in most of the organic solvent and also partially soluble in water, hence it is challenging to find a good dispersant. We use Pickering emulsion to encapsulate fatty amine in silicone oil and then coat the cores with a pH-responsive shell which can partially dissolve and because porous in acidic environment, thus the fatty amine can be release rapidly. We validate the rapid release in the acid solution by using bromophenol and also compared the neutralizing performance of free fatty amine and encapsulated fatty amine after high temperature treatment.
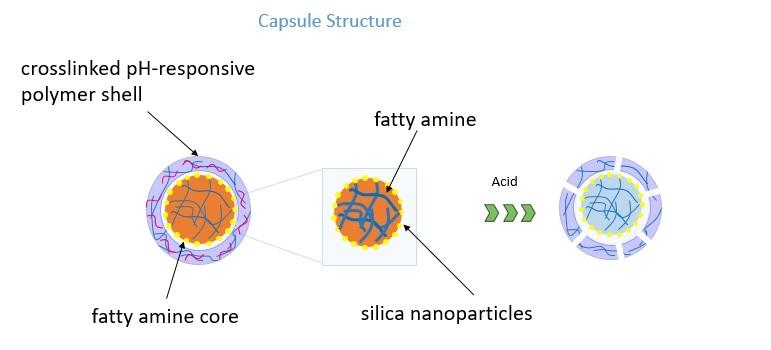
Figure3. The structure of the pH-responsive capsule. The core is crosslinked polymer containing fatty amine. The shell is pH-responsive shell. Upon contacting with acid. The pH-responsive component of the shell will dissolve and results in a porous shell. The fatty amine can be released rapidly.
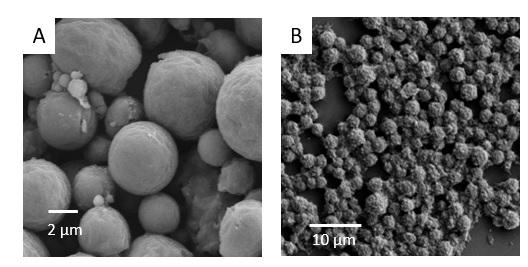
Figure4. The microscopic images of the structure of the pH-responsive capsules. A) microscopic image of the final particles. B) This microscopic picture shows that the surface of the core of the pH-responsive capsule is not as smooth as the surface of the final particles. Because the remaining silica particles are on the surface of the cores. The cores are made in the silicone oil by Pickering emulsion.
This work is in collaboration with Liangliang, Zhengwei and Dong.
