We use our expertise and our experimental tools to investigate the properties of biological materials and to study the behavior of cells. Much of our focus is on developing an understanding of the mechanical properties of biopolymer networks, formed by reconstituting proteins into gelled networks. These include networks of actin, microtubules, intermediate filaments, fibrin and collagen. We combine visualization of the network structure with probes of their mechanical properties to understand the nature of these properties. We also add molecular motors to the networks to investigate the properties of active gels. In addition, we extend our investigations to study the mechanical properties of cells and collections of cells. We also have an effort in understanding the growth and physical properties of biofilms.
Here are some current projects from our group:
|
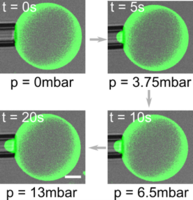
Impact of membrane-tethered polymers on lipid vesicle mechanics: The biophysical properties of lipid vesicles are important for their stability and integrity; they are also important for controlling the performance when these vesicles are used for drug delivery. Generally, the vesicle properties are determined by the composition of lipids used to form the vesicle. However, for a given lipid composition, they can also be tailored by tethering of polymers to the membrane. We develop a general method to functionalize lipid vesicles with synthetic polymers and polysaccharides and incorporate them on the outer membrane leaflet of giant unilamellar vesicles (GUVs). We investigate their effect on membrane mechanics using micropipette aspiration and membrane fluidity using fluorescence recovery after photobleaching. The results provide the potential means to study membrane-bound meshworks of polysaccharides similar to the cellular glycocalyx; moreover, they can be used for tuning the mechanical properties of drug delivery vehicles. Kevin Jahnke |
|
Engineering liposomes and lipid nanoparticles for drug delivery: The successful delivery of nucleic acids to cells is an important way to fight disease. It critically depends on the biophysical and chemical properties of the drug delivery vehicle. Liposomes and lipid nanoparticles are often employed as drug delivery vehicles because their properties can be meticulously fine-tuned. However, the search for the best possible modification for an efficient delivery is still ongoing. We functionalize the outer leaflet of the drug delivery vehicles with different polymers and investigate their effect on cellular uptake and transfection. The polymers encompass a wide range of properties that can affect the performance of the drug delivery vehicles. This work is a stepping stone for the development of more efficient drug delivery vehicles using controlled modifications. It also provides the means for the targeted delivery of drugs to specific cell types via polymer recognition. Kevin Jahnke, Chenjing Yang, Chunhuan Liu, Tiffany Chen |
 |
In-vivo study of Yielding and Post-yielding behavior of Cytoplasm and its linkage with the cytoskeleton: Cytoplasm, as an omnipresent component of the cells, is known to have finite Young’s modulus and resists deformation, while intracellular cargo transports as fast as micron per second is observed in cytoplasm, thus it must exists a transition from solid to liquid, so called yielding and post yielding behavior of the cytoplasm. We find that cytoplasm yields at 10^1 Pascal scale. The post yielding behavior may be modelled as non-Newton fluid. Further control experiment illustrates that both heterogeneity and microtubules significantly contribute to the yielding behavior: The resistance of cytoplasm has multiple resources, and the dynamic assembling and dissembling of the microtubules are essential for yielding. Also, the cytoplasm close to microtubule-organizing centre (MTOC) has higher resistance towards yielding. Our experiment can potentially enhance people’s understanding towards cargo transport and cell mechanics. Sijie Sun |
|
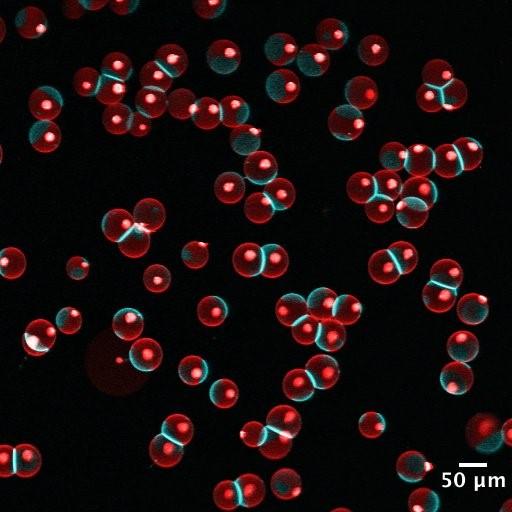
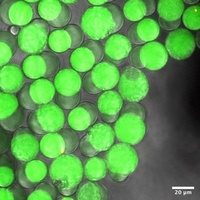
The interplay of phase behavior, membrane tension and membrane-membrane interaction in lipid vesicles: Lipid vesicles are aqueous volumes enclosed by lipid bilayers, which resemble the membrane backbone of cells and many intracellular organelles. Lipid vesicles have been used as an experimental model system to study membrane biophysics. However, traditional methods for vesicle generation depend on the self-assembly of lipid bilayers, which have shown poor control over the membrane composition, size distribution and structure of lipid vesicles, limiting the collection of quantitative, statistically sound data. To circumvent these problems, we have developed a microfluidics method for vesicle generation, which templates the formation of lipid vesicles on well-controlled double emulsions. The lipid vesicles made in this method have very narrow size distribution and uniform membrane composition. We use 3D reconstructed confocal microscopy to record the phase behavior and shape of lipid vesicles, and measure their membrane tension using micropipette aspiration. Combining these data, we are now investigating the rich interplay between membrane tension, phase behavior and membrane-membrane interaction. In the meanwhile, we are exploring robust methods to incorporate membrane proteins into the vesicles consistently. We hope this study can provide statistically sound data to further our understanding of lipid membrane biophysics. Anqi Chen |
|
Silk hydrogel formation within microfluidic droplets: Silk hydrogels are important for cosmetics, drug delivery and tissue engineering. We encapsulate silk fibroin into surfactant-stabilized water-in-oil droplets to study the kinetics of their formation. Droplet-based microfluidics allows a high-throughput screening of silk hydrogel formation inside individual compartments. Within one day at room temperature silk fibroin aggregates and forms a hydrogel. We study the particle size via the droplet diameter, silk fibroin concentration or ethanol content in the aqueous phase to complement our understanding of the gelation process. Kevin Jahnke |
|
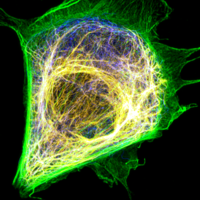
Studying the interplay between cytoskeleton filaments and cytoplasmic properties: The cytoplasm is the major component of live cells by volume, and its mechanical property plays a critical role in cell bioactivity, including mitosis, migration, and cargo transport. There is a constant interest in understanding the cytoplasm of a live cell from a mechanical perspective. It is known that the cytoskeleton network dominates the mechanical property of the cytoplasm. Cytoskeleton is an interpenetrating network composed of three filaments: F-actin, intermediate filament, and microtubules. In Weitz lab, we combine active microrheology with live cell imaging techniques to directly quantify the deformation of the cytoskeleton networks under load to better understand each filament's role and their interactions. Our research interest covers phase behavior, microrhoelogy, superresolution imaging and filament dynamics. Sijie Sun |
| Modulating GUVs towards enhanced mechanical versatility: Giant Unilamellar Vesicles (GUVs) are spherical capsules which have been used as model systems to study membrane biophysics, as cell membrane mimicking boundaries for bottom-up modular reconstitution studies, and as drug delivery carriers. Unlike the cell membrane, however, GUVs lack comparable mechanical versatility due to the two-dimensional fluidic nature of phospholipids and other lipids from which GUVs are assembled from. We aim to modify and modulate the contents of GUVs to achieve mechanically durable capsules. Few potential mechanisms we aim to explore to enhance mechanics of GUVs are: generating hybrid (polymer/lipid) vesicles, enriching membrane bilayer using transmembrane proteins, and mimicking the cell by assembling a cytoskeletal cortex bound adjacent to the GUV bilayer. Mechanical properties such as membrane bending rigidity, membrane viscosity and deformation recovery will be investigated using micropipette aspiration. Nadab Wubshet | |
|
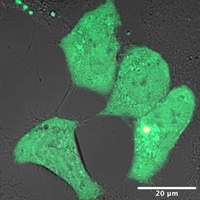
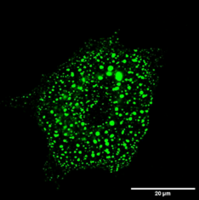
Liquid-liquid Phase Separation and Cell Cytoskeleton: Cytoskeletal filaments undergo assembly and disassembly is cells at very rates that are surprisingly fast. There are indications that cells sustain such fast rates by carrying out the assembly inside phase separated condensates where concentration of sub-units is much higher. I focus on the assembly of an intermediate filament called Vimentin, which is implicated in cancer metastasis. We study the properties of the precursors of Vimentin intermediate filaments (VIFs) to understand the role of phase separation in the assembly of VIFs. Figure.1 shows a cell with the VIF precursors appearing as green blobs of varying sizes. We carry out in vivo experiments on the precursors using known and novel reagents to probe their phase behavior as well as estimate their viscosity and surface tension. Arkaprabha Basu |
|
|
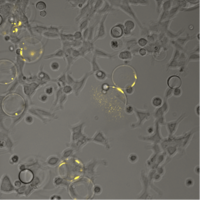
Microfluidic determination of lipid vesicle stiffness: The stiffness of lipid vesicles is important for engineering drug delivery vehicles and gaining fundamental insights into membrane biophysics. However, the determination of vesicle stiffness is mainly limited to three techniques: fluctuation spectroscopy, electrodeformation and micropipette aspiration. These suffer from very low throughput and lab-to-lab differences, which impedes a generalized and accepted catalogue of lipid vesicle bending rigidities and stretching moduli. We use microfluidics to develop a platform for the high-throughput screening of the mechanical properties of lipid vesicles. This will allow a standardized comparison between lipid compositions and decipher the impact of individual lipids on vesicle stiffness. Wenyun Wang, Kevin Jahnke |
|
Photodegradable capsules for local cargo delivery: The spatiotemporal control over the release of drugs is essential for investigating local responses of cells to chemical cues. However, classical methods like microneedles require to actively interfere with the sample, are low in throughput and cannot be used for 3D-tissues like organoids. Here, we employ microfluidics to form microcapsules that can be added to cell monolayers or tissues and locally degraded using light as a non-invasive external stimulus. Upon their degradation, they release their cargo which can then be taken up by cells. We use this approach to study the local response of cells in real-time. Moreover, we engineer capsules to induce apoptosis with full spatiotemporal control. Wentao Xu, Kevin Jahnke |
|
Shielding Biological Sensors of Force Propels Scarless Memory Repair and Regeneration: Human skin is a specialized mechanoresponsive interface, adapting to dynamic physical cues from embryonic development to lifelong external forces. Despite the recognized impact of the physical environment on cutaneous processes, the fundamental molecular mechanisms governing the skin's response to force remain elusive. Currently, the heart of this complex interaction is thought to be mechanotransduction, a process through which intracellular pathways convert mechanical cues into biochemical responses. Moreover, the extracellular matrix itself is increasingly recognized to mechanically regulate the spatiotemporal distribution of soluble and matrix-bound ligands, underscoring the importance of bidirectional crosstalk between cells and their physical environment. Notably, a structural hierarchy exists to maintain both cells and matrix in mechanical homeostasis, and dysregulation of this architectural integrity may underlie or contribute to scar formation. By delving into the study of mechanical stimulation and its intricate relationship with the mechanisms, we can pave the way for innovative therapies aimed at reducing scarring in wounds, thereby significantly improving the outcomes for patients in need of effective dermatological treatments. Di Suo |
Here are some previous projects from our group:
|
|
Microrheology in cells and networks: Cells are mechanical objects, with mechanics largely determined by their biopolymer cytoskeleton. We characterize local mechanics in cells and reconstituted biopolymer networks using active optical tweezer microrheology. We aim to determine the mechanical role of the biopolymers, as well as the effects of molecular motors and enzymes on the mechanics of the networks. Helen Wu, Mikkel Jensen, Ming Guo |
|
|
Mechanics of vimentin intermediate filament networks: Intermediate filaments (IF) make up a component of the cytoskeleton that has received much less attention than actin and microtubules. I am studying the mechanics of vimentin, a Type III IF, using bulk and microrheological techniques as well as traction force microscopy on single cells. I am interested in its contribution to cell stiffness and its role in cell mechanosensitivity. Helen Wu |
|
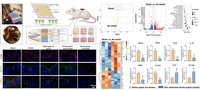
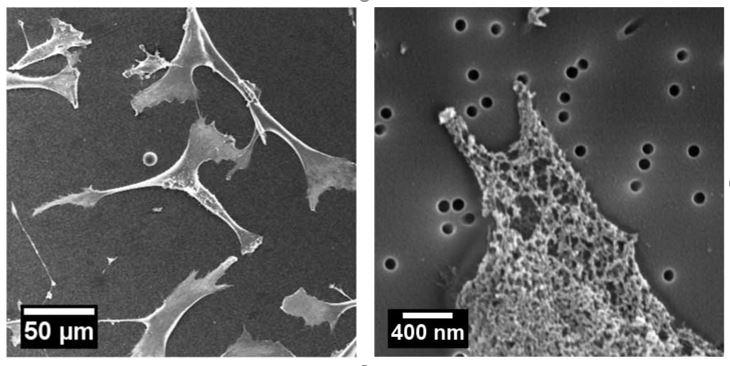
Decoupling the effects of nanopore size and surface roughness on the attachment, spreading and differentiation of bone marrow-derived stem cells: The nanopore size and roughness of nanoporous surface are two critical variables in determining stem cell fate, but little is known about the contribution from each cue individually. To address this gap, we use two-dimensional nanoporous membranes with controlled nanopore size and roughness to culture bone marrow-derived mesenchymal stem cells (BMSCs), and study their behaviors such as attachment, spreading and differentiation, as shown in Figure 1. We find that increasing the roughness of nanoporous surface has no noticeable effect on cell attachment, and only slightly decreases cell spreading areas and inhibits osteogenic differentiation. However, BMSCs cultured on membranes with larger nanopores have significantly fewer attached cells and larger spreading areas. Moreover, these cells cultured on larger nanopores undergo enhanced osteogenic differentiation by expressing more alkaline phosphatase, osteocalcin, osteopontin, and secreting more collagen type I. These results suggest that although both nanopore size and roughness can affect BMSCs, nanopore size plays a more significant role than roughness in controlling BMSC behavior. Jing Xia |
|
|
Mechanical properties of composite cytoskeleton networks: The cytoskeletal network is known to be responsible for maintaining cell mechanical integrity and determining cellular functions. We develop a method that enables us to reconstruct a three-component in vitro network composed of F-actin, intermediate filaments (vimentin filaments), and microtubules, which are the three fundamental cytoskeletal components. This composite is more physiologically relevant compared with any of the previously reported reconstituted cytoskeletal networks, which are composed of one or two components only. We investigate the structure and mechanical properties of this multi-component cytoskeletal network using several microscopy techniques as well as one- and two-point microrheology. We show that vimentin filaments hardly increase the network elastic modulus; however, they slow down the longitudinal motion of the polymers and significantly prolong the network relaxation time, by imposing configurational constraints within the network. These findings deepen our understanding of the mechanical role that vimentin filaments play in regulating cellular activities. Yinan Shen |
|
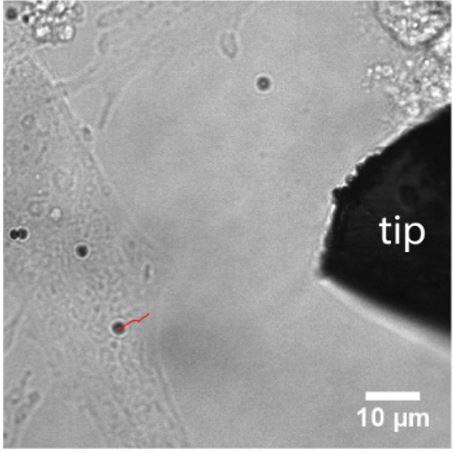
Design of electromagnetic tweezer for cellular mechanics studies: The mechanical properties of cell are very important for maintaining cellular integrity and fulfill their functions. However, there are only limited tools to investigate the mechanics of cells at submicron scale, such as atomic force microscope and optical tweezer, partially due to the limited force range available in each tool. Here, we develop an electromagnetic tweezer that can apply forces spanning several orders of magnitude, as shown in Figure 2. By controlling the electric current in the solenoid, the force can be varied from a few piconewton to hundreds nanonewton. This tool is further used to investigate the cell mechanics. By applied the magnetic force on the paramagnetic beads that’s attached on the cells, we can probe the mechanical properties of cells, as shown in Figure 3.Jing Xia |
