1. The interplay of phase behavior, membrane tension and membrane-membrane interaction in lipid vesicles
Lipid vesicles are aqueous volumes enclosed by lipid bilayers, which resemble the membrane backbone of cells and many intracellular organelles. Lipid vesicles have been used as an experimental model system to study membrane biophysics. However, traditional methods for vesicle generation depend on the self-assembly of lipid bilayers, which have shown poor control over the membrane composition, size distribution and structure of lipid vesicles, limiting the collection of quantitative, statistically sound data. To circumvent these problems, we have developed a microfluidics method for vesicle generation, which templates the formation of lipid vesicles on well-controlled double emulsions. The lipid vesicles made in this method have very narrow size distribution and uniform membrane composition. We use 3D reconstructed confocal microscopy to record the phase behavior and shape of lipid vesicles, and measure their membrane tension using micropipette aspiration. Combining these data, we are now investigating the rich interplay between membrane tension, phase behavior and membrane-membrane interaction. In the meanwhile, we are exploring robust methods to incorporate membrane proteins into the vesicles consistently. We hope this study can provide statistically sound data to further our understanding of lipid membrane biophysics.
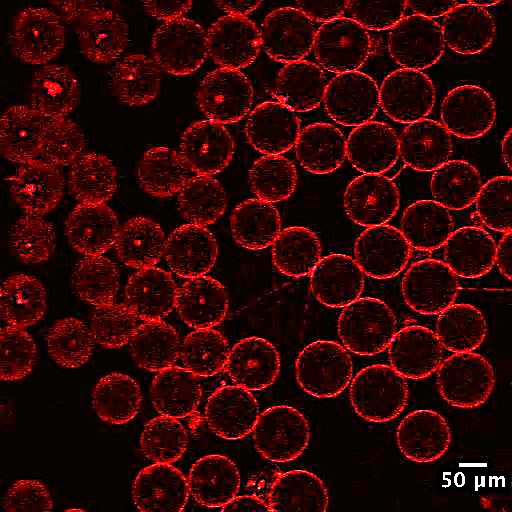
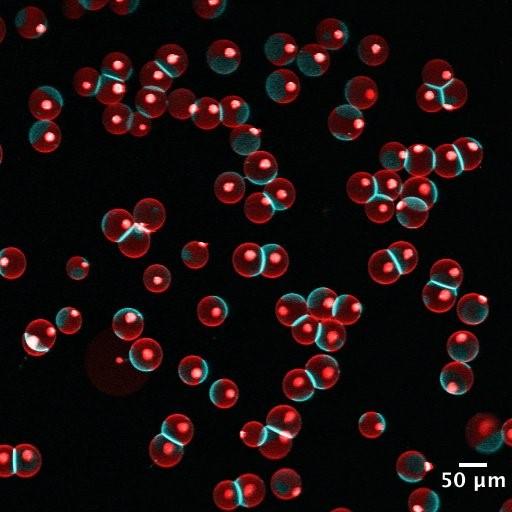
Figure 1. Fluorescent confocal images showing an example of the interplay between lipid phase and membrane-membrane interaction. a) Vesicles with membranes in a liquid disordered phase (red). No significant adhesion observed between neighboring vesicles. b) Vesicles with phase separated membranes in a liquid ordered phase (blue) and a liquid disordered phase (red). Significant adhesion and contact angle observed between neighboring vesicles. Adhesion is often, if not always, found in the liquid ordered phase.
2. Microfluidic encapsulation of mesenchymal stem cells in alginate hydrogel
Mesenchymal stem cells (MSCs) are a type of stem cells derived from bone marrow, umbilical cord and a few other tissues. Due to their wide therapeutic potential in tissue regeneration and immunoregulation, MSCs are currently regarded as the most promising candidate for new stem cell therapy. Some clinical experiments have shown promising results when using MSCs to treat systemic immune diseases. Despite their outstanding performance in some studies, consistent efficacy of MSC therapy is still hard to achieve. One of the biggest challenges that hinders the approval of MSC therapy is the short persistence time of MSCs in the host body. After administration in the host body, MSCs are usually lost within 2-3 days. This early cell loss is due to the lack of proper mechanical signaling during the transplantation process, as well as the attack of the host’s immune system. To overcome these problems, we develop microfluidics-based live cell encapsulation strategies to provide the injected cells with an extracellular substrate which at the same time can serve as a layer of protection against the host’s immune system. According to the required administration route, we can perform single- or multi-cell encapsulation in hydrogels of various structures and mechanical properties. Subcutaneous injection of MSC-encapsulating alginate microcapsules made with our method have shown promising persistence time in mouse experiments.
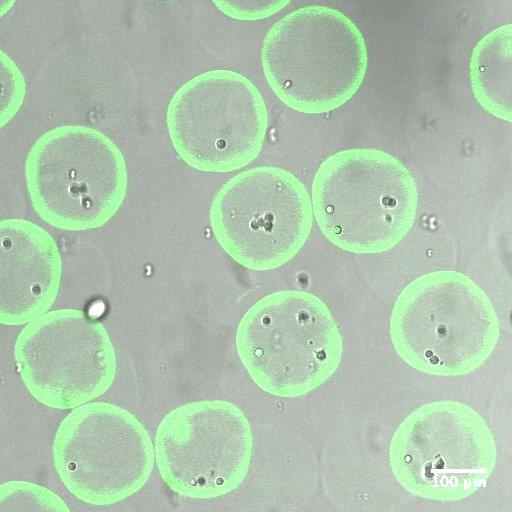
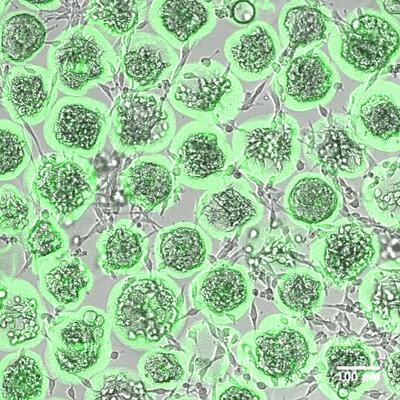
Figure 2. Overlaid fluorescent confocal images and wide field images of MSCs encapsulated in alginate microcapsules. a) Immediately after microfluidic encapsulation b) Microfluidic encapsulation followed by 5 days of in vitro culture. Alginate molecules are fluorescently labeled to show the capsule structure.
Contact: Anqi Chen anqichen@g.harvard.edu
