Materials Physics using Complex Droplets Generated with Microfluidics
Large quantities of sophisticated drops are controllably generated with microfluidics. These structures have varying degrees of complexity in both their internal and external structure. Elegant and mesmerizing, these 'designer' emulsions can be exploited to pave new ways of understanding complex phenomena in physics, chemistry, biology and material science. For example, with physics, arrays of multi-component drops can be designed for probing how collective behavior arises from interactions of simple constituents inside drops across a network of drops in one, two and three dimensions. Such systems could provide understanding of interactions in strongly correlated materials; at present, these interactions lack exact analytical solutions and solutions using numerical analysis are impossible to solve computationally in dimensions greater than two. Furthermore, two and three-dimensional crystalline structures can be encapsulated inside another drop to study packing of drops under confinement.
Encapsulated drops can be engineered as micro-reactors, smart sensors or as flowbots. Spherical batteries or encapsulated bubbles for contrast agents with functional shells can also be generated. Even stable non-spherical microcapsules with separate compartments are generated for transport of pharmaceuticals in the body. Remarkable and adaptive, these new materials cannot be made by any other means than microfluidics; they are promising sources of fascinating new physical phenomena that are both fundamentally and technologically important.
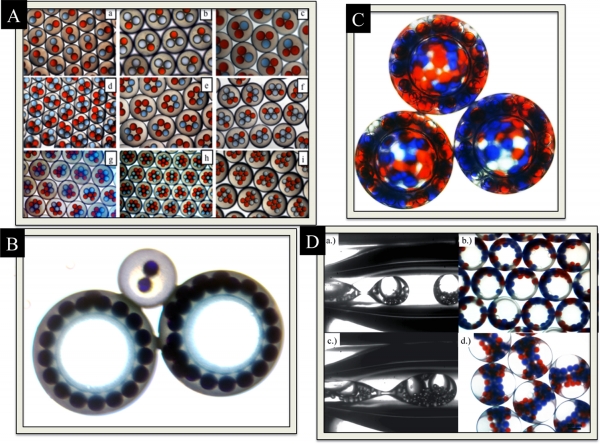
Figure 1 – Double Emulsions Generated with Drop-Based Microfluidics. Figures taken from Adams et al. Soft Matter (2012)
Asymmetric Fission of a Water Drop
When a fluid droplet breaks-off from a thinning fluid thread, the thickness of the fluid neck becomes vanishingly small at the point of snap-off leading to a singularity. Minimization of surface energy drives the snap-off mechanism by reducing the surface area of the thread.
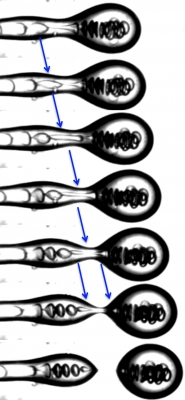
Below and to the right are time lapse images of asymmetric fission of a water drop inside an oil drop. Before the outer oil droplet breaks-off due to the Plateau -Rayleigh Instability, an inner water droplet is trapped in the thinning fluid thread. As the tread becomes thinner and thinner, the water drops deforms and stretches before splitting asymmetrically into two.
Stimuli Responsive Materials with Droplet-Based Microfluidics: Triggerable Coalescence or Release of Cargo
Triggered Coalescence. Controlled coalescence of inner drops inside double emulsions, drops inside of drops, is useful for triggering chemical reactions inside drops with the outer drop serving as a reaction vessel. Using double emulsions as micro-reactors is one of our reasons for encapsulation different types of inner drops inside another drops. Coalescence of inner drops is triggered with heat from a heat gun as seen in this gif. The outer drop is made of was using a melt emulsification technique. As the wax melts, the inner aqueous droplets are free to rotate and move around until they come into contact with one another. Prior to drops coalescing, a small bridge is formed between the drops. This bridge is noticeable in Fig. 3.
Figure 3 –Triggerable temperature sensitive microcapsules for coalescence.
Triggered Release. Double emulsions, drops inside of drops, are useful as carriers of drugs and other macromolecules. The mechanical properties of the shells, such as their elasticity, permeability and selectivity, are tunable using different material compositions during their microfluidic production. Here we show a microcapsule with a bio-compatible wax shell composed of different triglycerides. As the shell is heated, the wax shell melts and expands releasing cargo containing different aqueous drops as seen in the Fig. 4.
Figure 4 –Triggerable temperature sensitive microcapsules for release.
Foams with Monodispersed Bubbles
Foams are trapped pockets of gas in liquids or solids; their structure is a source of fascination to almost everyone that comes across them-from scientists, engineers, chefs, and baristas to young children and adults playing with soap bubbles. What is the optimal shape of bubbles in a foam when the bubbles all have the same volume? Lord Kelvin, in 1887, was the first to ask this question as a way to understand the simplest and lowest energy state of how bubbles pack when having equal volumes in a foam.
Figure 5a – Generation of encapsulated bubbles.
Here we show the formation of a two-dimensional foam as seen in Figure. 5b. The pockets of gas are encapsulated in a thin, flexible shell composed of nanoparticles and toluene. The encapsulated bubbles are placed on a glass slide as they exit from a microfluidic device. The liquid shell solidifies over time causing the foam to transition from a liquid to solid structure.
Figure 5b – Formation of a two dimensional foam from encapsulated bubbles.
As the shells of the encapsulated bubbles come into contact with each other, the shape of the bubbles transition from spherical to polyhedral shapes as seen in Figure 5b. The boundaries between the polyhedral shapes are called Plateau borders. Plateau borders are important for fluid drainage and are useful in describing the stability of foams. With microfluidics, we can controllably generate foams that are either liquid or solid and containing different size bubbles.
Flowbots with Microfluidics
Caterpillar double emulsions. This work is done in collaboration with Jiawai Yang of Zhigang Suo’s Group. Traditional rigid body robots are limited in their ability to interact with their environment and maneuver in highly congested environments, such as the human body. These cylindrically-shaped double emulsions, drops inside of drops, wiggle and buckle after they detach from the nozzle and flow through a microfluidic capillary device. Our goal is to utilize flowbots to detect and cure diseases in the human body, like something from the science fiction movie Fantastic Voyage, by loading them with drugs and sensors.
Droplet Imaging Velocimetry (DIV): Visualizing Fluid Flow with Ultra-Low Surface Tension Droplets
Figure 7 – Droplet imaging velocimetry
The usual methodology of visualizing fluid flow is with tracer particles using particle imaging velocimetry (PIV); however, tracer particles are not ideal in droplet based microfluidic experiments since they have to be very small, typically nanometers, to avoid clogging devices or to avoid altering the flow dynamics in micron size channels. Thus, they are difficult to see even with an optical microscope. Furthermore, fluorescent tracer particles can be used but this requires a fluorescent microscope to detect their fluorescence. Even still, the signal contains a lot of noise, and thus achieving an accurate flow profile is still challenging. In this new technique, named droplet-imaging velocimetry (DIV), water droplets with ultra-low surface tension are used to directly map the velocity profile of fluid flow in an immiscible fluid. Here we show the flow profile during jetting as double emulsions are generated in a microfluidic device as seen in the upper left image. This technique provides valuable information about fluid properties of drop formation.
For example, to study fluid flow inside the Rayleigh-Plateau Instability, we use DIV and reduce the interfacial tension between inner drops and the middle phase. Poiseuille flow, or pipe flow, is clearly visual on the left of the video shown above. Between pinch-off and Poiseuille flow, the fluid slows down as seen in the absence of curvature of the inner drops. As the outer drop pinches off, and the flow substantially slows down to a near halt, the inner drops are no longer affected by the flow; their shape responds to the absence of flow by having a spherical geometry. These drops inside the oil drop that pinches off start to coil because they contain charged surfactants.
For more information, please visit my personal page.






