Dendrimers, Polymers and Particles – Macromolecular Materials Synthesis
Maximilian A. Zieringer, Ph.D.
The main target of my research is the development of carrier systems for the efficient encapsulation and triggered release of active substances for the practical application in agriculture, drug delivery, cosmetics, and construction. Design, synthesis, and modification of polymers offer unlimited opportunities to create carrier systems with unique features. Polymers are synthesized or modified to precisely control the release of encapsulated actives from microcapsules and microparticles. In addition, microfluidic fabrication techniques facilitate manipulating the structural composition and size of microcarrier systems; allowing the generation of systems that meet highest requirements..
Polymer Particles as Multi-Active Carriers
We use a perfluorinated-dendrimer–dye complex that stabilizes microbubbles as a novel pore-forming agent. Microfluidics enables us to produce monodisperse emulsions containing a polymer matrix material, a model active, and the perfluorinated complex; upon drying, the emulsions form porous microspheres as shown in Figure 1. This porosity causes the encapsulated model active to be released faster than from non-porous microspheres. Moreover, because of the fluorous features of the pores, we can also attach an additional guest molecule to the pores which is released with a profile that is distinct from that of the encapsulated active. These porous microspheres can encapsulate and controllably release multiple actives; this makes them valuable for applications such as drug delivery and imaging.
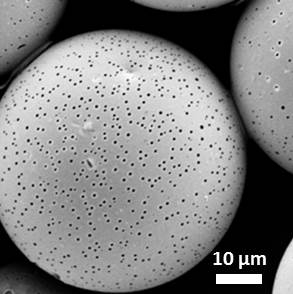
Figure 1. Scanning electron micrographs of porous PLA microspheres made with dendrimer-dye complex.
Microcapsule Shells for Long-Term Storage and Triggered Release of Encapsulated Actives
This project is in collaboration with Nick J. Carroll.
Polymeric microcapsules consist of a core and consolidated shell. Typically, the core is either liquid or solid and contains actives. The shell consists of a polymeric network and is used as a barrier to keep the actives separated from the exterior. Microcapsules hold great potential for applications involving the encapsulation and triggered release of actives for applications in agriculture, drug delivery, oil industry, and encapsulation of food ingredients. However, the leakage of actives from capsules is typically observed and presents a technological challenge for the practical application of capsule technologies. Our goal is to enhance practical applications of microcapsules by fabricating cross-linked shells from customized polymer precursors that will prevent encapsulated actives from leaking prior to triggered release; we fabricate microcapsules with chemical inertness, long-term stability independent of external pH, and high mechanical stability. Additionally, our shell material allows capsule fabrication with high encapsulation efficiency and allows for cargo diversity (hydrophobic or hydrophilic actives).
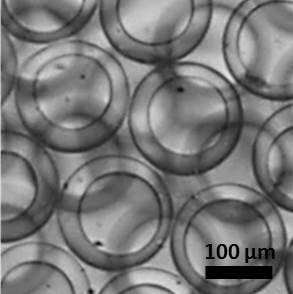
Figure 2. Microcapsules for long-term storage of actives.
Nanocapsules for the Encapsulation of CT Contrast Agents
Iodine based contrast agents have deleterious effects on patients with significant renal impairment and/or previous severe reactions to iodinated contrast media due to its nephrotoxicity and hypersensitivity/allergic reactions. Also, such agents are avoided in patients receiving thyroid treatment with radioactive iodine to avoid interference with iodine uptake. To avoid these undesirable effects, we develop nanocapsules for the encapsulation of CT contrast agents for in vivo imaging. Through various alterations in the fabrication process, we are able to generate nanocapsules with unimodular size distribution. Ideally, these capsules will be cleared from the blood without release of encapsulated contrast agents. Currently, we study the pharmacokinetics of these capsules in various relevant in vitro and in vivo models.
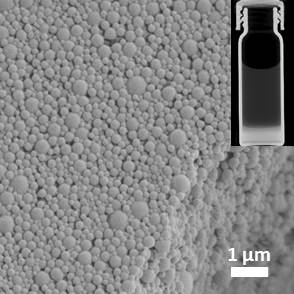
Figure 3. Scanning electron micrograph of nanocapsules. Insert: Micro-CT scan of encapsulated Iodinated contrast agent (bottom layer) in water (top layer).
Chemically Resistant Microfluidic Devices
Chemically resistant microfluidic devices allow the unlimited use of reagents and solvents and therefore extend the scope of microfluidics in synthesizing new materials. Fluorinated polymers such as fluorinated ethylene propylene (FEP), perfluoroalkoxy (PFA), or perfluoropolyethers (PFPE) show exceptional solvent resistance, are chemically and biologically inert, and are highly suitable for microfluidic devices because of their optical transparency and their mechanical strength. We developed various fabrication processes for rapid prototyping of microfluidic devices made from fluorinated polymers. Concurrently, we develop methods to build microfluidic devices that can withstand extreme reaction conditions such as high temperatures and high pressures.
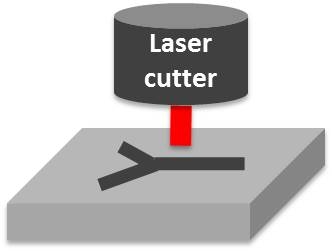
Figure 4. Illustration of rapid prototyping of chemically resistant devices using a laser cutter.
Controlling Polymer Composition through Polymerization in Microfluidic Devices
Block-copolymers and gradient polymers with well controlled molecular structure are traditionally produced by ionic and living radical polymerization. Ionic polymerization requires good purity of the reagents, living radical polymerization requires somewhat expensive additives that in certain cases are not desired in the polymeric product. Therefore, these technologies are not widely applied in producing commodity chemicals. This project aims at producing block-copolymers and gradient polymers with well-defined structure through inexpensive free radical polymerization in microfluidic channels. The spatial confinement and the exquisite control over residence times in microchannels allow manipulating the structural composition of growing polymer-chains. In a continuous process, at subsequent positions along the microchannel, radicals are generated, polymer chains are allowed to grow, and finally, other monomers are added to the growing polymer radicals flowing down the channel. So far, this method reliably yields block-copolymers with a high degree of control over the composition of each block.
